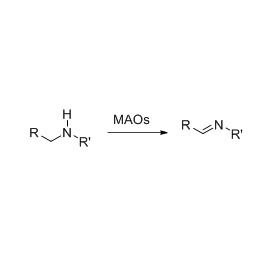
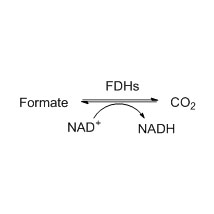
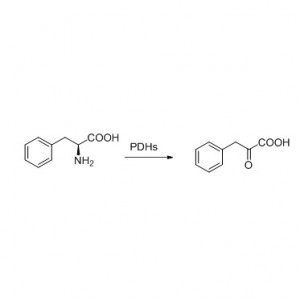
Amidase (AMD)
Enzymes:Su ne macromolecular nazarin halittu catalysts, mafi yawan enzymes sunadaran gina jiki
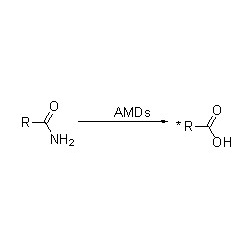
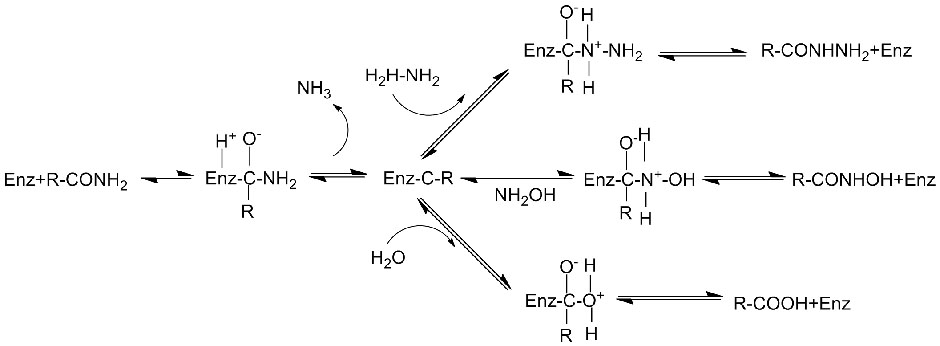
Amidase:Catalyze da hydrolysis na daban-daban endogenous da na waje aliphatic da aromatic amides ta hanyar canja wurin wani acyl kungiyar zuwa ruwa tare da samar da free acid da ammonia.Hydroxamic acid da sauran kwayoyin acid ana amfani dasu sosai azaman magunguna saboda sune abubuwan haɓaka haɓaka, maganin rigakafi da masu hana ƙari.Ana iya raba amidases zuwa nau'in R da nau'in acylases na S bisa ga abin da ke haifar da stereoselectivity.
Baya ga catalyzing da hydrolysis na amides, amidase kuma iya catalyze acyl canja wurin halayen a gaban co-substrates kamar hydroxylamine.
Amidase tare da maɓuɓɓuka daban-daban suna da ƙayyadaddun ƙayyadaddun ƙayyadaddun ƙayyadaddun ƙayyadaddun abubuwa, wasu daga cikinsu na iya yin hydrolyze aromatic amides kawai, wasu daga cikinsu na iya yin hydrolyze kawai aliphatic amides, wasu kuma hydrolyze α-ko ω-amino amides.Yawancin amines suna da kyakkyawan aiki na catalytic kawai don acyclic ko sauki aromatic amides, amma ga hadaddun aromatics, heterocyclic amides, musamman amides tare da ortho substituents, gabaɗaya a cikin aiki (kawai ƴan enzymes suna nuna mafi kyawun tasirin tasiri).
Na'urar catalytic:
| Enzymes | Lambar samfur | Lambar samfur |
| Enzyme Foda | ES-AMD-101~ ES-AMD-119 | saitin amidases 19, 50 MG kowane 19 abubuwa * 50mg / abu, ko wasu adadi |
| Kit ɗin Nuna (SynKit) | Saukewa: ES-AMD-1900 | saitin amidases 19, 1 MG kowane abubuwa 19 * 1 MG / abu |
★ High substrate takamaiman.
★ Karfin chiral selectivity.
★ High juyi yadda ya dace.
★ Kadan kayan masarufi.
★ Sharuɗɗan ɗaukar hankali.
★ Abokan Muhalli.
➢ Ya kamata a gudanar da gwajin gwajin enzyme na musamman saboda ƙayyadaddun ƙayyadaddun kayan, kuma a sami wani enzyme wanda ke haifar da maƙasudin maƙasudin tare da mafi kyawun tasirin kuzari.
➢ Kada a taɓa hulɗa da matsanancin yanayi kamar: babban zafin jiki, high / low pH da kwayoyin kaushi tare da babban taro.
➢ Yawanci, tsarin amsa ya kamata ya haɗa da substrate, maganin buffer (Mafi kyawun halayen pH na enzyme).Co-substrates irin su hydroxylamine ya kamata su kasance a cikin tsarin amsawar acyl.
➢ Ya kamata a ƙara AMD ta ƙarshe cikin tsarin amsawa tare da mafi kyawun amsa pH da zazzabi.
➢ Duk nau'ikan AMD suna da mafi kyawun yanayin amsawa daban-daban, don haka kowane ɗayansu yakamata a ƙara yin nazari akai-akai.
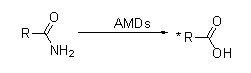
Misali 1(1):
Ayyukan hydrolysis zuwa daban-daban Amide Substrates
| Substrate | takamaiman ayyuka μmols min-1mg-1 | Substrate | takamaiman ayyuka μmols min-1mg-1 |
| Acetamide | 3.8 | ο- OH benzamide | 1.4 |
| Propionamide | 3.9 | p- OH benzamide | 1.2 |
| Lactamide | 12.8 | ο-NH2benzamide | 1.0 |
| Butyramide | 11.9 | p-NH2benzamide | 0.8 |
| Isobutyramide | 26.2 | ο- Toluamide | 0.3 |
| Pentanamide | 22.0 | p- Toluamide | 8.1 |
| Hexanamide | 6.4 | Nicotinamide | 1.7 |
| Cyclohexanamide | 19.5 | Isonicotinamide | 1.8 |
| Acrylamide | 10.2 | Picolinamide | 2.1 |
| Metacrylamide | 3.5 | 3-Phenylpropionamide | 7.6 |
| Prolinamide | 3.4 | Indol-3-acetamide | 1.9 |
| Benzamide | 6.8 |
An dauki matakin a cikin 50mM sodium phosphate buffer bayani, pH 7.5, a 70 ℃.
| Amides | Hydroxylamine | Hydrazine |
| Acetamide | 8.4 | 1.4 |
| Propionamide | 18.4 | 3.0 |
| Isobutyramide | 25.0 | 22.7 |
| Benzamide | 9.2 | 6.1 |
An dauki matakin a cikin 50mM sodium phosphate buffer bayani, pH 7.5, a 70 ℃.
Abubuwan da suka shafi reagent: amides, 100 mM (benzamide, 10 mM);hydroxylamine da hydrazine, 400 mM;0.9 μM.
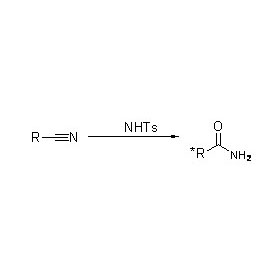
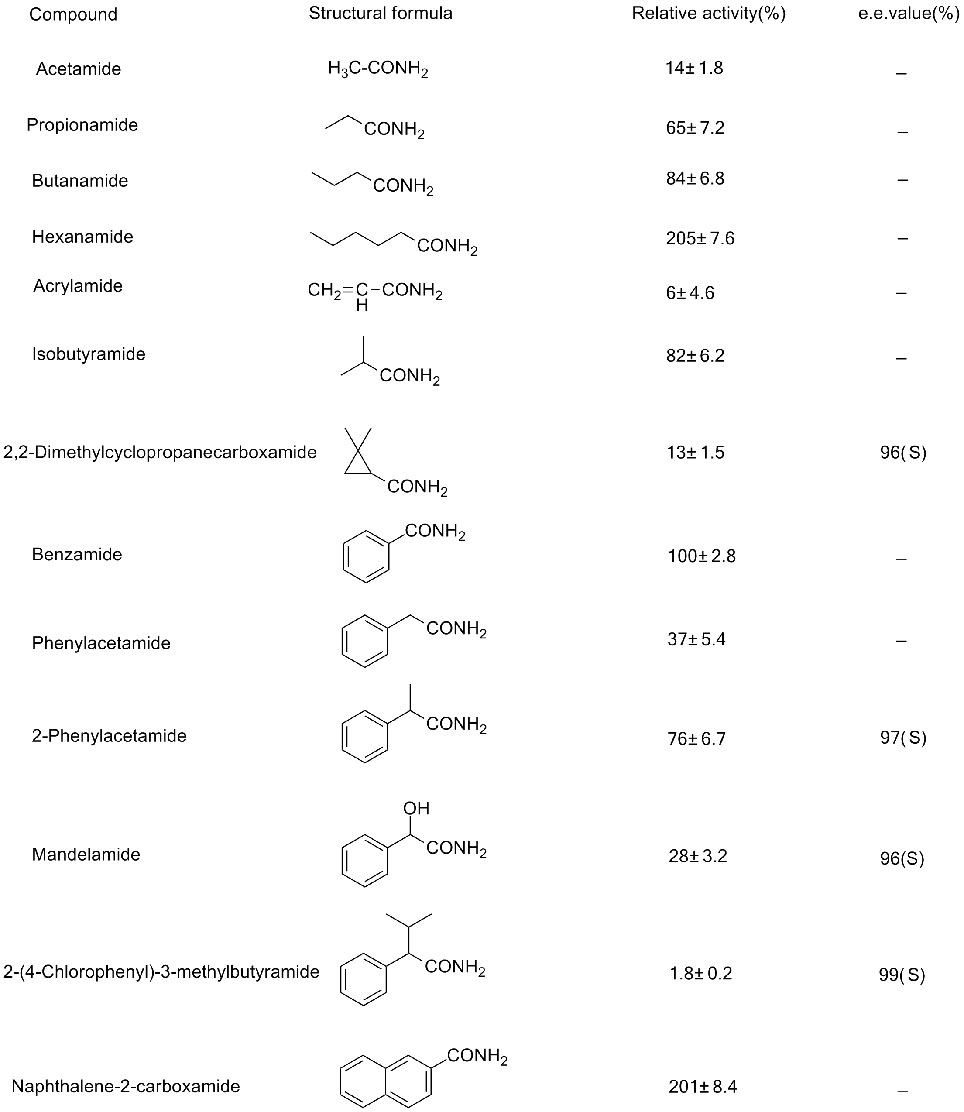
Misali 2(2):
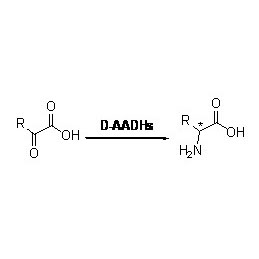
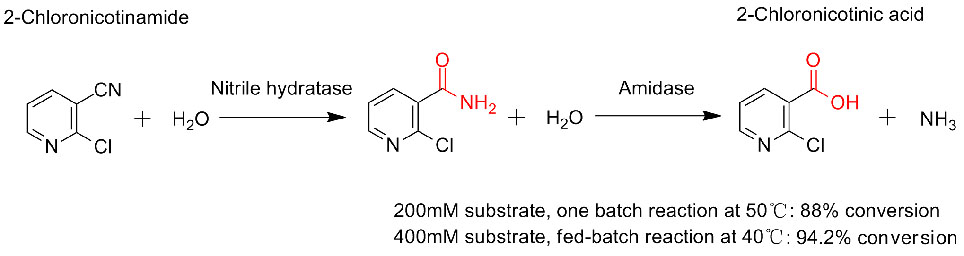
Misali 3(3):
1. D'Abusco AS, Ammendola S., et al.Extremophiles, 2001, 5:183-192.
2. Guo FM, Wu JP, Yang LR, da dai sauransu.Tsari Biochemistry, 2015, 50 (8): 1400-1404.
3. Zheng RC, Jin JQ, Wu ZM, et al.Bioorganic Chemistry, 2017, Akwai akan layi 7.